This is good and wrote a number of articles with my focus being to having a scanning solution for FDA recalls and this is a big step forward here as there’s information that can be attached to “real time” data. I would still like to see this pursued for consumer recall information and one day, who knows as being able to scan a bottle of Tylenol would represent some real value for me as a consumer. Here’s yet one more technology to where the 2D bar codes can be edible and put right on the pill and so my prediction is we are not done here yet with bar codes. The 2D bar code business was flop in other areas outside of healthcare as folks only used it to basically advertise and market, completely overlooking “useful” place for them. In New York, they tried to put bar codes on garbage trucks, well that didn’t work and I had this vision of a consumer chasing a garbage truck to scan the code..not (grin).
I would still like to see this pursued for consumer recall information and one day, who knows as being able to scan a bottle of Tylenol would represent some real value for me as a consumer. Here’s yet one more technology to where the 2D bar codes can be edible and put right on the pill and so my prediction is we are not done here yet with bar codes. The 2D bar code business was flop in other areas outside of healthcare as folks only used it to basically advertise and market, completely overlooking “useful” place for them. In New York, they tried to put bar codes on garbage trucks, well that didn’t work and I had this vision of a consumer chasing a garbage truck to scan the code..not (grin).
Bar Codes for Drugs Is Not Dead, They are Edible Now, The Battle Against Counterfeit Drugs With Tagging the Pills…
A while back I did a summary of my bar code ideas and you can find it here along with an article I wrote a few years back on how being able to access information on a device that had been recalled could have saved a life. Now with the upcoming device bar codes, the FDA has addressed this. I keep this flipping magazine article on the blog here as well so you may have run across it. Here’s my summary page which also includes showing how the bar codes can be used to tag and put medical data into PHRs and EHRs as well. Razcode has a nice implementation on using the 2D barcodes for e-prescribing too. Here’s a video below from my archives on how HealthVault had the 2D bar codes connected and more videos at the link.
the upcoming device bar codes, the FDA has addressed this. I keep this flipping magazine article on the blog here as well so you may have run across it. Here’s my summary page which also includes showing how the bar codes can be used to tag and put medical data into PHRs and EHRs as well. Razcode has a nice implementation on using the 2D barcodes for e-prescribing too. Here’s a video below from my archives on how HealthVault had the 2D bar codes connected and more videos at the link.
“Auth Tag” – Mobile Microsoft Bar Code Tags Using a Smartphone To Scan for Two Factor Authentication Giving Users Digital Tokens
RAZCODE (Microsoft Tags) Using Smart Phones to authenticate MDs When e-Prescribing Controlled Substances
Healthcare Bar Code Discussions/Ideas
Razcode one of the companies doing this with encrypted technologies and funny now that the FDA announcement is out I see a lot of hits on that 2 year old archive post for use as Medical Tokens. I’m sure Bob at Razcode might be getting some inquiries.
If you want to dig in a little more, there’s a Microsoft technology patent to make the bar codes tamper resistant as well.
Microsoft Receives Patent-Techniques to Create Counterfeit and Tamper Resistant Labels Using Fiber Optic Strands-Bar Codes Getting Closer for Drug/Device Recalls?
As you know Microsoft has left the 2D business but they are still alive and well out there with another company taking over.
RAZCODE (Microsoft Tags) Using Smart Phones to authenticate MDs When e-Prescribing Controlled Substances
Here’s what the FDA image shows on how the bar codes will look and appear.
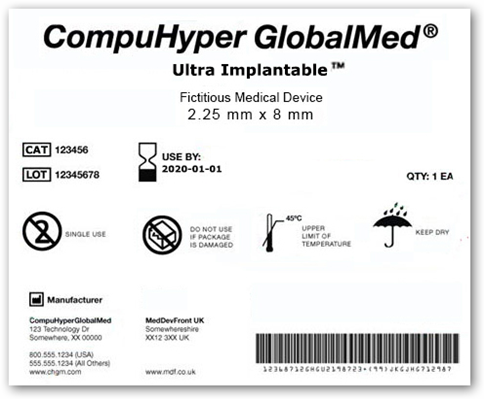
Of course it will take a while to get these into production but the first step is here after a long wait and a lot of work. You will be able to scan the device yourself if you want and get information with your phone. So if you have an implant and you want to check on it, make sure you have the bar code and you can do it yourself. There’s still a commenting period here so based on input there could be additional news coming out. They did it right with the GUDID data base and within 3 years of the final publication all Class 3 devices will need the bar code.
Class One devices have a few more years to get their bar codes in place. Actually the FDA is being extremely lenient with implementation times in my opinion. Here’s another article I wrote a few years back on having the bar codes on insulin pumps.
Like I said earlier everyone was kind of stupid just trying to use the bar codes for advertising and it flopped like I said it would as the real focus is providing value for consumers and not pumping your own company ego but that’s the big mistake everyone made, not knowing where technologies are beneficial and just wanting to beat their own chest.
Insulin Pump Problems and Recalls – FDA Get Those Tags Out There So Consumers Can Identify Them Easily with Cell Phones
Stay tuned as I’ll keep this topic covered! You can even search the Medical Quack and dig up a bunch more here as I had quite a collection of posts on bar code use technologies in healthcare. BD
The Food and Drug Administration (FDA) has released a final rule requiring that most medical devices distributed in the United States carry a unique device identifier, or UDI. It also applies to certain combination products that contain devices and to devices licensed under the Public Health Service (PHS) Act (e.g., donor screening assays).
A UDI system has the potential to improve the quality of information in medical device adverse event reports, which will help the FDA identify product problems more quickly, better target recalls and improve patient safety. In developing the proposed UDI system, the FDA worked closely with industry, the clinical community and patient and consumer groups, and conducted four pilot studies.
As part of the UDI system, the FDA is also creating the Global Unique Device Identification Database (GUDID) which will include a standard set of basic identifying elements for each device with a UDI. Most of this information will be made available to the public so that users of a medical device can easily look up information about the device. The UDI does not indicate, and the database will not contain, any information about who uses a device, including personal privacy information.
The FDA has issued Global Unique Device Identification Database (GUDID) - Draft Guidance for Industry (PDF - 3.6 MB) to give labelers an overview of the GUDID. This draft guidance is designed to help labelers prepare to submit information to the GUDID by describing key GUDID concepts such as accounts, user roles, the device identifier record life cycle, package configurations, and the GUDID data attributes and descriptions. The FDA encourages the submission of comments and suggestions regarding the draft GUDID guidance. Comments may be submitted electronically or by mail according to the directions contained in the draft guidance within 60 days of the Federal Register publication of the notice announcing its availability.
http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/UniqueDeviceIdentification/default.htm



0 comments :
Post a Comment