 The system monitors ECG, heart rate, respiration rate and activity and was developed in coordination with the Mayo Clinic. They look like band aids that are place on 3 parts of the body. The data is sent wirelessly to a smartphone to the Preventice Care platform. The physician can log on at any time on a computer or mobile device to get the data. When you look at the partner page interesting that “payers” are included here and of course they would want behavioral type analytics and do they sell de-identified information? The devices and platforms are meant to monitor non lethal cardiac arrhythmias so this is monitoring and not a monitoring system with a big sense of urgency, that is unless something becomes urgent. BD
The system monitors ECG, heart rate, respiration rate and activity and was developed in coordination with the Mayo Clinic. They look like band aids that are place on 3 parts of the body. The data is sent wirelessly to a smartphone to the Preventice Care platform. The physician can log on at any time on a computer or mobile device to get the data. When you look at the partner page interesting that “payers” are included here and of course they would want behavioral type analytics and do they sell de-identified information? The devices and platforms are meant to monitor non lethal cardiac arrhythmias so this is monitoring and not a monitoring system with a big sense of urgency, that is unless something becomes urgent. BD From the Website:
“Developed in collaboration with Mayo clinic, this FDA-cleared system uses sophisticated algorithms to support remote monitoring for individuals
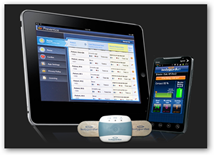 with non-lethal cardiac arrhythmias. BodyGuardian RMS allows physicians to monitor key biometrics outside of the clinical setting, while patients go about their daily lives. Important physiologic data is securely collected by a small, wearable body sensor and transmitted to physicians. It maintains a constant connection between patients and their care teams. BodyGuardian RMS detects and monitors non-lethal cardiac arrhythmias in ambulatory patients, when prescribed by a physician or other qualified healthcare professional. The BodyGuardian System continuously records, stores and periodically transmits the following physiological data to a remote computer server for up to 30 days at a time.”
with non-lethal cardiac arrhythmias. BodyGuardian RMS allows physicians to monitor key biometrics outside of the clinical setting, while patients go about their daily lives. Important physiologic data is securely collected by a small, wearable body sensor and transmitted to physicians. It maintains a constant connection between patients and their care teams. BodyGuardian RMS detects and monitors non-lethal cardiac arrhythmias in ambulatory patients, when prescribed by a physician or other qualified healthcare professional. The BodyGuardian System continuously records, stores and periodically transmits the following physiological data to a remote computer server for up to 30 days at a time.”
PreventiceTM, Inc., a Minnesota company that develops mobile health applications, announced Monday that the U.S. Food and Drug Administration (FDA) has approved its BodyGuardian Remote Monitoring System (RMS) for use by hospitals and clinics.
Developed in collaboration with Mayo Clinic, the BodyGuardian System allows remote monitoring for individuals with cardiac arrhythmias. The BodyGuardian System will allow physicians to monitor their patients while they go about their daily lives, Preventice said Monday.
A small body sensor attached to the patient's chest collects important data, including the patient's ECG, heart rate, respiration rate, 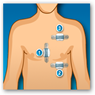 and activity level. That data then can be transmitted to physicians via mobile phone technology. The remote monitoring can create a constant connection between patients and their care teams. FDA approval allows Preventice to market and sell BodyGuardian to hospitals and clinics. Although not yet commercially available, the BodyGuardian RMS will be prescribed by a physician when diagnostic and post-procedure monitoring is needed. Preventice said it anticipates the system will be commercially available by the end of this year.
and activity level. That data then can be transmitted to physicians via mobile phone technology. The remote monitoring can create a constant connection between patients and their care teams. FDA approval allows Preventice to market and sell BodyGuardian to hospitals and clinics. Although not yet commercially available, the BodyGuardian RMS will be prescribed by a physician when diagnostic and post-procedure monitoring is needed. Preventice said it anticipates the system will be commercially available by the end of this year.
http://www.startribune.com/business/169177526.html



0 comments :
Post a Comment